Related Stories
Business & Markets
TUSPAN syrup is the newest addition to our OTC portfolio based on extract of ivy leaf.
Progress
Strongly committed with local communities and people wellness, we have been integrating...
Development of the medicinal products, medical and regulatory activities are supported by the following departments:
Under the leadership of Regulatory Affairs and the participation of other departments, the registration documentation of medicinal product is prepared in accordance with current regulatory requirements in the country, the dossiers in electronic format (eCTD) are prepared for registration of our new drugs via Community and national procedures, for renewal of granted marketing authorizations and changes in documentation.
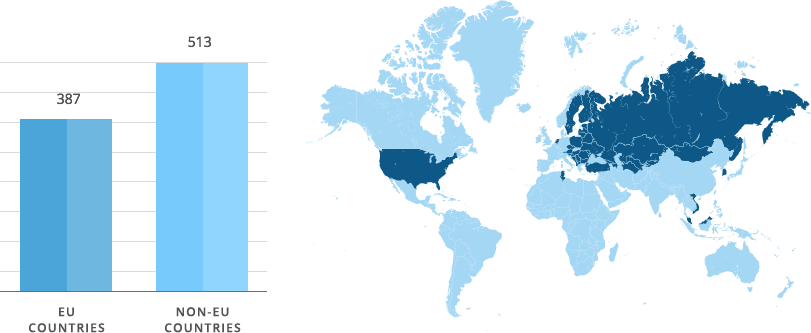
Our objective is to register new Sopharma AD products faster and following the most cost-effective approach. That is why our regulatory strategy for registration of new products via a decentralized procedure is a priority. Positive assessment of our dossiers by 14 European national competent authorities shows that we develop qualitative, safe and efficient medicinal products and present to the Regulatory authorities documentation that meets current regulatory requirements.
Sopharma AD has 900 granted Marketing Authorisations around the world.